Vinay Parthan, VP R&D, Medication Delivery Solutions
Klaus Hoerauf, VP Medical Affairs, Medication Delivery Solutions
The COVID-19 pandemic is the latest reminder that healthcare workers around the world face hazards related to their work every day, both visible and invisible. In many cases, medical devices and equipment are the primary means to prevent injury or disease. Some of the most seminal safety innovations used in the United States today seem simple, even obvious, but as recently as the 1980s, U.S. healthcare workers accepted exposure to syringe needles and undetected hazardous substances as risks associated with their jobs. These dangers impacted workers with physical, emotional and monetary costs and contributed to shortened average lifespans.
Major health crises, such as the rise of Hepatitis B and C and HIV/AIDS catalyzed new preventive standards for healthcare worker safety, such as the Occupational Safety and Health Association's guidance for bloodborne pathogens, and a deeper level of focus on safety device innovations. But safety should not only be the afterthought of a crisis. It’s our responsibility to serve healthcare workers and patients by working to advance safety across the continuum of care, every day. Throughout the last century and this one, our R&D teams have helped BD pave the way in safety device innovation, leveraging extensive experience with healthcare operations and challenges, and applying the most cutting-edge technology to safety-enhancing innovations.
For National Safety Month this June, we’re sharing the story of innovations that required deep clinical knowledge, a commitment to quality and R&D excellence. Our dedication to pioneering technology has readied us to meet these critical moments through innovations that deliver incredible impact keeping healthcare workers safe.
Innovations to Prevent Needlestick Injury and Blood-Exposure
Less than 40 years ago, it was common to see needles stuck into a nurse’s scrubs or into the side of hospital bed for temporary “safekeeping.” Nurses and doctors accepted that, some days, they may be stuck by an exposed needle. Patients and cleaning staff also suffered needlestick injuries. Then, Hepatitis B and C and HIV/AIDS completely changed how the industry and society viewed the threat of needlesticks.
Before OSHA adopted its bloodborne pathogens guidance in 1991, engineers at BD began tackling the problem of needlestick injuries. We developed needlestick safety devices to reduce needlestick injuries and the ensuing infections and disease from bloodborne pathogens. In fact in 1988, BD introduced the first syringe with a built-in feature to protect healthcare workers from needlestick injuries. The
3cc BD Safety-Lok™ Syringe was designed with a protective shield that moves forward and locks in place, eliminating the risk of healthcare workers sticking themselves accidentally with a contaminated needle during recapping. This innovation was the harbinger of many new technologies to help protect healthcare workers and their patients, such as safety catheters, sharps containers and phlebotomy devices to reduce the risk of unintended blood exposure.

This innovation continues to be a priority today, as we launch new technologies like our straight passive safety IV catheter, designed to prevent needlestick injuries and increase insertion success, thanks to design features that allow a familiar insertion technique on all gauge sizes and an ergonomic push tab for improved insertion control and rapid adoption. We’re invested in developing needle technologies that minimize the risk of healthcare worker exposure to blood, such as our BD Instaflash™ Needle with a passive safety system that shields the needle as it’s withdrawn.
Building safety measures into even a seemingly simple device requires advanced R&D, design and manufacturing know-how. We leverage simulated use studies to provide usability insights to ensure high quality products performed consistently, as well as automated manufacturing expertise to scale production to meet demand.
Hazardous Drug Exposure Prevention Innovation
One of the leading invisible threats to safety comes through exposure to hazardous drugs in the form of vapors, liquids and surface contamination over months and years. The National Institute for Occupational Safety and Health (NIOSH) reports that 8 million U.S. healthcare workers per year are potentially exposed to hazardous drugs, such as chemotherapy treatments. These substances put workers at a higher risk to develop both acute and chronic side effects, including cancer, DNA aberrations, fertility issues and damage to internal organs.
As just one example among numerous studies proving the adverse effects, oncology nurses identified in the Danish cancer registry from 1943-1987 revealed a “significantly increased risk” of leukemia, attributed to exposure to antineoplastic drugs.
Much like with needlestick dangers, for a long time, the technology clinicians needed to protect themselves didn’t exist. Then in 1994, Carmel Pharma, which BD later acquired, pioneered the first closed-system drug transfer device. Today, there are several leading innovations to reduce exposure for healthcare workers who prepare and administer hazardous drugs or work in areas where they are used.
Closed-system drug transfer devices
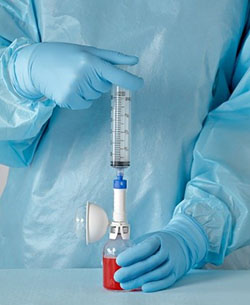
To solve for the challenge of hazardous drug exposure, our R&D teams had to identify opportunities to rethink the clinical workflow. Engineering safety into the drug transfer process meant that we needed to introduce an air-tight connection under multiple uses. Leveraging industry-leading materials expertise, our R&D team integrated the right materials for the need—selecting, for example, rubber for membranes to help ensure for leakproof conditions. Knowing that you can’t undo an exposure, and that healthcare workers need a device that will work reliably under a variety of everyday conditions, our engineers employed strong systems engineering skills for multiple design requirements, such as tear strength, elasticity, creep, hardness and swell ratio to maximize performance.
Faster hazardous drug contamination testing
Even with the utmost caution, accidents happen. That’s why it was critical to consider not only preventing the exposure in the transfer process, but also how to detect hazardous drug contamination that may be present on surfaces.

In the early days of testing for the existence of hazardous drugs on surfaces, turnaround time for results was as long as a month, by which time exposure damage was already done. We knew there was a better way. Our engineers revolutionized testing for hazardous drug surface contamination with our BD® HD Check System, taking detection time from weeks to under 10 minutes for select hazardous drugs. They integrated diverse technologies to enable the assay and placed a strong focus on usability to consistently sample a surface. This meant facilities could check that their safety measures were working in near real-time to better protect healthcare workers by preventing unnecessary exposure.
The future of healthcare worker safety innovation
Advances in safety technology today have made healthcare settings much safer, but safer is not safe enough. OSHA reported 221,400 work-related injuries and illnesses in U.S. hospitals in 2019, almost twice the rate of the private industry as a whole. It’s why continued safety innovation across the entire workflow is essential.
Additionally, med-tech companies increasingly have a duty to develop these devices without the impetus of a crisis. It’s critical that we talk to our healthcare customers on a regular basis, observe how they do their work and bring insights back to expert R&D teams. A company with the scale and depth of expertise that BD has can see a care organization’s workflow processes through a unique lens and identify problems the organization may not even be aware of. Even when developing system components that require sophisticated technological and manufacturing capabilities, close customer interaction throughout product development and an unwavering focus on human factors and user experience can help us ensure that devices have a real impact for healthcare workers and are simple to use.
We’re also innovating to make existing solutions more accessible around the world. Too many of the devices we’ve described are not available in most parts of the world and are not used in clinical care settings.
As we look to the future, we’re identifying ways to use data analytics, automation and robotics to predict and prevent safety problems. Imagine a care setting with cameras observing healthcare worker practices that can analyze and predict problems in time to warn clinicians before a negative outcome occurs. Or imagine auto-fulfillment stations at pharmacies in which advanced robotics mean healthcare workers never have to touch hazardous drugs. At BD, we’ll continue to push the frontiers of design, materials science, sterilization, formulation chemistry, metallurgy, sensing devices and global access as we continue our pursuit of advancing the world of healthTM and healthcare worker safety.
*Tests for select hazardous drugs. Surfaces with contamination at or above the limits of detection have 95% specificity and sensitivity.
Subscribe to receive BD blog alerts