Ask a parent what’s most important to them right now, and you’ll likely hear: Returning children to school safely. That priority—and that focus on safety—is shared by teachers and academic administrators alike.
The MOT Charter school in Delaware has found in the BD Veritor™ Plus System a solution to help face this challenge.
“We were a bit nervous about it,” admitted Mitch Weiss, Director of Student Services as MOT Charter School. “This kind of program is, of course, entirely new to us—to our staff, our teachers, our students and their parents. But implementing the testing program has been easy and has given us all a sense of confidence, comfort and helped us return to something like normal. We had promised our families that we would do whatever it took to get students safely back in school and this was a significant step towards meeting that goal.”

MOT Charter is one of twenty schools participating in a state-wide program developed jointly by the Delaware Department of Education (DOE) and Delaware Health and Social Services (DHSS) to leverage testing to safely reopen schools by providing – at state expense – BD Veritor™ analyzers and SARS-CoV-2 (COVID-19) tests. The teams at both MOT Charter and the DOE are enthusiastic about their success and are eager to share their experiences to encourage use of the BD Veritor™ at other schools across Delaware and more broadly.
MOT Charter is a 1,385 student K-12 public charter program across two campuses (K-8 and 9-12). Currently, about half of its students are back in school with the rest remaining virtual. They had been working remotely throughout the fall and wanted to find the best way to safely reopen in the spring.
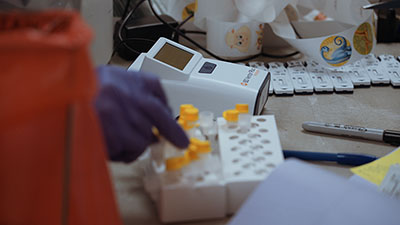
“We wanted to bring our students back in a way that was easy for them and that gave our parents peace of mind,” said Weiss. “With careful planning, the cooperation of our teachers and staff, and using the BD Veritor™, we’ve been able to do just that. It meets two specific needs for us. It both demonstrably makes our campus safer, and it also makes our campus feel safer. Both needs had to be met for families to send their children and for staff to feel confident about being in school.”
MOT started using the BD Veritor™ just before the winter break by familiarizing parents and children with the testing process.
“It feels just like picking your nose,” joked one of the MOT teachers, a silly and fun way to help lessen children’s—and parents’—fears and concerns.

“We believe that the ease of the process was crucial in helping show parents that we are sensitive to their worries,” said Weiss. “The buzz on social media about how easy it was really helped spread the word.”
In mid-January, MOT began in-school testing (in addition to temperature screening at the doors, staggered schedules and requirements for masks and social distancing). “Depending on the age group, we bring a cart around to the classrooms for students to come to one at a time or have a central location where swabs can be collected in smaller groups,” Weiss explained. “It only takes about a minute for each student.”
The samples are then taken to the nurse’s office or processed right in the hallway on the BD Veritor™. Test results are available in just 15 minutes. If a child tests positive, they are discretely and sensitively collected from the classroom and remain in the nurse’s area while their parents are called to come pick them up. The school nurse’s priority is to meet the needs of that child and their family.
The student’s classmates take recess while the classroom is cleaned and any close contacts (students and staff within six feet for 15 or more minutes) are identified. After that, the rest of the instructional day proceeds as normal and at the end of the day, the school follows its normal COVID communication plan with families.

Reflex (confirmation) testing via PCR molecular testing is encouraged, and students must test negative or complete a quarantine period before returning to school.
“Some parents who were initially not planning to have their children return to school have changed their minds and opted in after seeing how easy testing is,” added Weiss. “Our teachers are very positive about it as well.”
To participate in testing, schools obtain a CLIA waiver certificate, a process which, Mitch notes, was not seen as a significant hurdle. The fact that Delaware schools are required to have onsite nurses has also been helpful, though several of the staff conducting testing at MOT do not have medical backgrounds. The processes MOT Charter have coordinated require administration by six staff members across the two schools to collect samples, run tests and report results. The reporting requirement is, MOT confirms, the most challenging, and BD has several options for facilitating automated reporting.
“The MOT program is exemplary, and we are very hopeful that more schools across Delaware will adopt similar programs,” said Dr. Rick Pescatore, Chief Physician, Associate State Medical Director, Delaware Division of Public Health. “Since the state is not mandating a specific approach, and a COVID-19 testing requirement is not in place, flexibility is very important. At this point, about 30 schools across three large school districts and several charters have opted-in to the program, and we’re looking forward to sharing best practices to make it even easier as new schools join in.”
Learn more at BDVeritor.com
About the BD Veritor™ Plus System for Rapid Detection of SARS-CoV-2 Assay
The BD Veritor™ Plus System for Rapid Detection of SARS-CoV-2 Assay has not been cleared or approved by FDA but has been authorized by FDA under an Emergency Use Authorization (EUA) for use by authorized laboratories. This product has been authorized only for the detection of proteins from SARSCoV-2, not for any other viruses or pathogens, and this product is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Act, 21 U.S.C. § 360bbb-3(b)(1), unless the declaration is terminated or authorization is revoked sooner.
The intended use of the BD Veritor™ Plus System for Rapid Detection of SARS-CoV-2 assay only includes those who are suspected of COVID-19 by their health care providers within the first five days on the onset of symptoms.
-
This product has not been FDA cleared or approved; but has been authorized by FDA under an EUA for use by authorized laboratories
-
This product has been authorized only for the detection of proteins from SARS-CoV-2, not for any other viruses or pathogens; and
-
This product is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug and Cosmetic Act, 21 U.S.C § 360bbb-3(b)(1), unless the declaration is terminated or authorization is revoked sooner
Testing is limited to laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C §263a, that meet the requirements to perform moderate, high or waived complexity tests. This test is authorized for use at the Point of Care (POC), i.e., in patient care settings operating under a CLIA Certificate of Waiver, Certificate of Compliance, or Certificate of Accreditation.
Subscribe to receive BD blog alerts