Press releases
| Su | Mo | Tu | We | Th | Fr | Sa |
|---|---|---|---|---|---|---|
| Su | Mo | Tu | We | Th | Fr | Sa |
|---|---|---|---|---|---|---|
-
Jul 7, 2021New online portal effectively aggregates, customizes and streamlines purchasing of essential reagents to accelerate bold research and clinical innovation
BD (Becton, Dickinson and Company) (NYSE: BDX), a leading global medical technology company, today announced the commercial release of a new bdbiosciences.com web and eCommerce site, an entirely...
-
Jun 30, 2021BD also announced plans to host an Investor Day
BD (Becton, Dickinson and Company) (NYSE:BDX) announced today that it will report its financial results for the third quarter of fiscal year 2021 on Thursday, August 5, 2021. BD will issue a press...
-
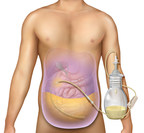
Jun 23, 2021PeritX™ Peritoneal Catheter System is the first and only at-home system indicated for malignant and non-malignant ascites drainage in the United States.
BD (Becton, Dickinson and Company) (NYSE: BDX), a leading global medical technology company, today announced it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for...
-
Jun 22, 2021
BD (Becton, Dickinson and Company) (NYSE: BDX), a leading global medical technology company, today issued its 2020 Sustainability Report, highlighting significant achievements in environmental,...
-
Jun 11, 2021
USA Swimming, the national governing body for the sport of swimming in the United States, and BD (Becton, Dickinson and Company) (NYSE: BDX), a leading global medical technology company, have...
-
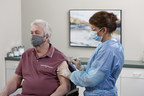
Jun 8, 2021Company Now Supplying More Than 40 Countries for Pandemic Vaccination Campaigns
BD (Becton, Dickinson and Company) (NYSE: BDX), a leading global medical technology company, today announced that it has received pandemic orders for needles and syringes totaling 2 billion...
-
Jun 2, 2021Becomes the First Medical Technology Company Authorized as a CVE Numbering Authority
BD (Becton, Dickinson and Company) (NYSE: BDX), a leading global medical technology company, today announced that it has become the first medical technology company authorized as a Common...
-
May 26, 2021
BD (Becton, Dickinson and Company) (NYSE: BDX), a leading global medical technology company, today announced that it will present at the following upcoming virtual investor conferences during the...
-
May 25, 2021BD Surgiphor™ sterile wound irrigation system delivers simplified sterility and helps hospitals comply with national and international guidelines[1,2]
BD (Becton, Dickinson and Company) (NYSE: BDX), a leading global medical technology company, announced today the availability of BD Surgiphor™ Sterile Wound Irrigation System, the first and only...
-
May 18, 2021New plant will produce drug delivery devices, employ 150 people in 2024 and up to 600 when full operations start in 2030
BD (Becton, Dickinson and Company) (NYSE: BDX), a leading global medical technology company, will build a €165 million ($200 million USD) high-tech manufacturing facility in the city of...