Expanded Collaboration Agreement to Make Less Invasive Blood Sample Collection More Convenient and Patient Centered
AUSTIN, Texas and FRANKLIN LAKES, N.J., May 11, 2022 /PRNewswire/ -- Babson Diagnostics, a science-first, health care technology company, and BD (Becton, Dickinson and Company) (NYSE: BDX), a leading global medical technology company, today announced the expansion of a strategic partnership to move blood sample collection into new care settings, including enabling patients to collect blood samples at home for diagnostic testing.
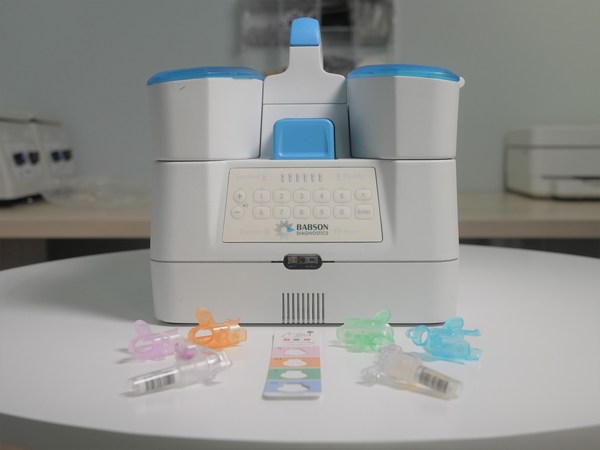
Since 2019, the two companies have collaborated to create a capillary blood collection and testing system, now in advanced development, to enable laboratory-quality, small-volume capillary blood collection at retail settings by team members with no previous experience in blood collection. Plans under the expanded agreement include continuing research and development to enable self-collection, mobile services and at-home collection. Babson and BD also will expand the types of blood tests that are possible through small-volume blood collection beyond primary care-oriented tests, and they also plan to develop diagnostic tests for pediatric use.
"Today's agreement expands our long-standing partnership with BD and builds on our shared passion of making convenient blood testing accessible to all, not only in the U.S., but also globally," said David Stein, chief executive officer of Babson Diagnostics. "Extending the medical home is critical in today's health care environment. We believe that retail convenience is perfect for today's consumer, but because the Babson service is well-suited to many settings with no need for a phlebotomist and an easier collection experience, we see many opportunities for future expansion."
Over this long-term collaboration, Babson and BD are advancing development of the blood testing ecosystem, which includes BD's next generation capillary collection technology and Babson's proprietary automated sample-handling and analytical technologies. These have been designed to work together to enable blood testing that requires only one-tenth the sample volume of traditional venipuncture methods without sacrificing quality, accuracy, or the number and types of tests that are possible.
"This is a paradigm-shifting solution that addresses multiple unmet needs within the current health care system," said Brooke Story, president of Integrated Diagnostics Solutions for BD. "Because it is less invasive and more convenient than the traditional venous blood draw method, capillary blood collection may lead to an improved patient experience, which in turn could help health care providers see better compliance among patients for routine blood testing."
BD brings 70 years of specimen management experience to the partnership and is a leader in blood collection technology, including the development of the new, state-of-the-art capillary collection devicei. Babson provides deep instrument analyzer expertise and is building an ecosystem for blood collection in the pharmacy setting as well as designing the systems and workflows to analyze small volume capillary blood in a central lab. Babson continues to work with local and national pharmacy chains to conduct extensive clinical studies of its service platform in preparation for commercial launch.
About Babson Diagnostics
Babson Diagnostics is a health care technology company rooted in science reimagining the entire diagnostic blood testing experience. Babson's mission is to make routine blood testing less invasive, more convenient and affordable, empowering people to take charge of their health.
Babson will bring medically accurate blood testing to the retail pharmacy by using patented technologies and a first-of-its-kind ecosystem that requires only one-tenth the sample volume of traditional venipuncture methods without sacrificing quality, accuracy, or menu breadth.
The company has received key patents in the United States, European Union, and China related to its unique end-to-end technological ecosystem and the ability to maximize the clinical utility of microsamples of blood collected from a fingertip. In addition, Babson has fully validated a broad set of miniaturized assays that are ready for commercial use in its CLIA-certified laboratory.
Babson, based in Austin, Texas, was founded by individuals with deep experience in healthcare, diagnostics, engineering, and laboratory technologies. It is named in honor of Art Babson, whose legacy of scientific innovation and excellence is the foundation on which the company is built.
About BD
BD is one of the largest global medical technology companies in the world and is advancing the world of health by improving medical discovery, diagnostics and the delivery of care. The company supports the heroes on the frontlines of health care by developing innovative technology, services and solutions that help advance both clinical therapy for patients and clinical process for health care providers. BD and its 75,000 employees have a passion and commitment to help enhance the safety and efficiency of clinicians' care delivery process, enable laboratory scientists to accurately detect disease and advance researchers' capabilities to develop the next generation of diagnostics and therapeutics. BD has a presence in virtually every country and partners with organizations around the world to address some of the most challenging global health issues. By working in close collaboration with customers, BD can help enhance outcomes, lower costs, increase efficiencies, improve safety and expand access to health care. For more information on BD, please visit bd.com or connect with us on LinkedIn at www.linkedin.com/company/bd1/ and Twitter @BDandCo.
|
Contacts: |
|
|
Babson |
|
|
Joe Foster |
|
|
Babson Diagnostics Media |
|
|
323-572-5361 |
|
|
BD Media |
BD Investor Relations |
|
Troy Kirkpatrick |
Francesca DeMartino |
|
VP, Public Relations |
SVP, Head of Investor Relations |
|
858.617.2361 |
201.847.5743 |
i The capillary collection device is an investigational device under 21 C.F.R. 812 and requires additional studies to make any definitive conclusions about safety or efficacy
SOURCE BD (Becton, Dickinson and Company)