FRANKLIN LAKES, N.J., Jan. 5, 2023 /PRNewswire/ -- BD (Becton, Dickinson and Company) (NYSE: BDX), a leading global medical technology company, today released findings from a survey conducted by The Harris Poll, between Nov. 14-16, 2022, among 872 U.S. women ages 18 to 64 years old, that indicate a significant gap in women's knowledge about the primary causes of cervical cancer as well as the most effective means of prevention.
Despite being one of the few cancers that is almost entirely preventable, according to the American Cancer Society (ACS) i, every year in the United States, 14,000 women are diagnosed with cervical cancer, and more than 4,000 women die from it.
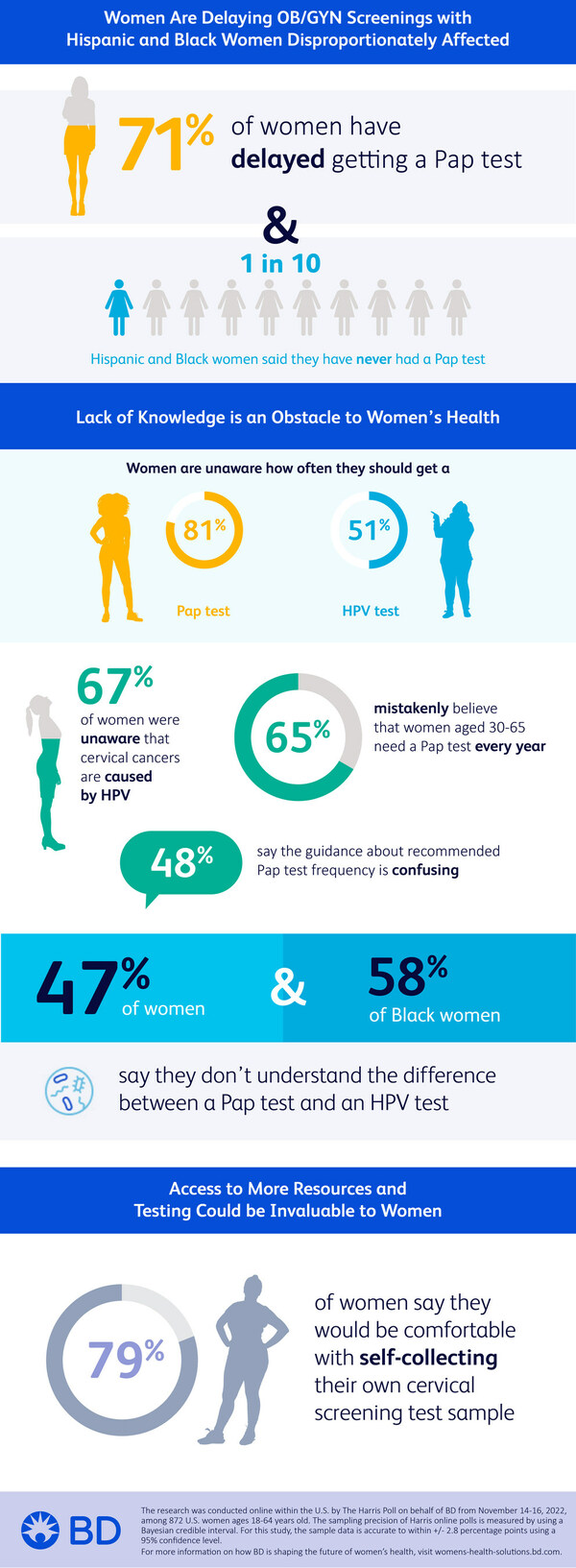
Women Are Delaying OB/GYN Screening; Hispanic and Black Women Disproportionately Affected
The online survey of U.S. women ages 18 to 64 found that 71% of respondents have delayed getting a Pap test, the screening that looks for precancers or cell changes on the cervix. Precancerous cells can turn into cervical cancer if they are not treated appropriately. Specifically, 15% of American women say their last OB/GYN visit for a routine care or check-up was more than three years ago, with nearly one in 10 (9%) saying they have never had a Pap test.
About one in 10 Hispanic and Black women say they have never had an OB/GYN visit for routine care (12% of Hispanic women and 8% of Black women vs. 3% of non-Hispanic white women) and similar proportions say they have never had a Pap test (12% of Hispanic women and 13% of Black women vs. 6% of non-Hispanic white women). When asked why they have delayed getting a Pap test, Hispanic women are more likely to say they were embarrassed (22% vs. 4% of Black women and 9% of non-Hispanic white women); afraid it would hurt (18% vs. 9% of non-Hispanic white women); or they didn't have an OB/GYN (21% vs. 11% of non-Hispanic white women).
Most American women, 75% in fact, say one of their resolutions for 2023 will be to get on track with their annual health screening appointments, like OB/GYN visits.
"Racial and ethnic minorities, rural residents, sexual and gender minorities and those with limited English proficiency often face cultural, economic and geographical factors that preclude them from obtaining critical health screenings, including Pap and HPV tests," said Brooke Story, worldwide president of Integrated Diagnostic Solutions for BD. "Being that January is Cervical Cancer Awareness Month, there is no better time to analyze the sentiment women hold around such screenings. The survey results underscore that lack of knowledge is one of the biggest barriers to receiving timely screening. We need more patient-centered communications to educate everyone, including and especially marginalized and underserved groups, in addition to providing greater access to critical diagnostic tools and services."
Lack of Knowledge is an Obstacle to Women's Health
While 91% of American women say they are knowledgeable about women's health in general, fewer report being knowledgeable about more specific aspects, such as how often women their age should get a Pap test (81% unaware) or HPV test (51% unaware). HPV tests screen patients for HPV, the virus that is thought to be responsible for nearly all cervical cancers, according to the U.S. Centers for Disease Control and Prevention (CDC).
The study found that 67% of American women were unaware that almost all cervical cancers are caused by HPV. Overall, 47% of American women say they don't understand the difference between a Pap test and an HPV test, with Black women (58%) more likely to agree with this than non-Hispanic white women (44%).
Similarly, 66% of American women did not know that nearly all sexually active men and women get HPV at some point in their lives, while 61% did not know there are different types of HPV strains. A majority of American women (55%) mistakenly believe that Pap tests screen for a variety of STDs, while 67% mistakenly believe that women aged 30 to 65 need a Pap test every year. However, The American College of Obstetricians and Gynecologists (ACOG)ii iii and U.S. Preventive Services Task Force (USPSTF)iv recommends screening begin at 21 years of age, with Pap testing every three years; and average-risk individuals aged 30 years and older screen every five years with primary HPV testing or co-testing. The ACSv recommends screening begin at age 25 with primary HPV screening.
Nearly four out of five American women (77%) say they defer to their OB/GYN to recommend how often they should get a Pap test, and 48% say the guidance about recommended Pap test frequency is confusing. Younger women are more likely to agree that they have no idea how often they're supposed to get a Pap test (46% for ages 18 to 34 and 47% for ages 35 to 44 vs. 24% for women ages 45 to 64).
Access to More Resources and Testing Could Be Invaluable to Women
The study found that 79% of American women say they would be interested in using a self-collection kit for HPV or cervical cancer screening, an alternative form of screening that allows women to collect their own specimen in private, at a time and place where they are most comfortable.
Younger women cited they are more likely to use self-collection for HPV or cervical cancer because it might be less expensive (38% vs. 28% of women ages 45 to 64); it might hurt less than a traditional Pap test (21% vs 9% of women ages 45 to 64); or they don't have health insurance (18% vs. 8% of women ages 55 to 64).
When citing reasons why they might be interested in a self-collection kit, Hispanic women are more likely than non-Hispanic white women to say they don't have health insurance (24% vs. 12%); or they live too far away from any health care providers (12% vs. 6%).
"As a gynecologist with more than three decades of clinical experience, I have seen firsthand how cervical cancer screenings can save women's lives," said Dr. Jeff Andrews, vice president of Medical Affairs, Integrated Diagnostic Solutions for BD. "Delivering effective, accessible screenings and improving the quality of medical care, regardless of race, geographic location or socioeconomic status, will empower women, and ultimately, work towards eliminating cervical cancer in our lifetime. Organizations like the American Cancer Society are joining with the White House Cancer Moonshot to promote educational outreach to women, awareness about HPV screening and to encourage adoption of self-collection for primary HPV screening."
The research was conducted online within the U.S. by The Harris Poll on behalf of BD from November 14-16, 2022, among 872 U.S. women ages 18-64 years old. The sampling precision of Harris online polls is measured by using a Bayesian credible interval. For this study, the sample data is accurate to within +/- 2.8 percentage points using a 95% confidence interval.
For complete survey data file, please contact mela.sera@bd.com.
BD is one of the largest global medical technology companies in the world and is advancing the world of health by improving medical discovery, diagnostics and the delivery of care. The company supports the heroes on the frontlines of health care by developing innovative technology, services and solutions that help advance both clinical therapy for patients and clinical process for health care providers. BD and its 77,000 employees have a passion and commitment to help enhance the safety and efficiency of clinicians' care delivery process, enable laboratory scientists to accurately detect disease and advance researchers' capabilities to develop the next generation of diagnostics and therapeutics. BD has a presence in virtually every country and partners with organizations around the world to address some of the most challenging global health issues. By working in close collaboration with customers, BD can help enhance outcomes, lower costs, increase efficiencies, improve safety and expand access to health care. For more information on BD, please visit bd.com or connect with us on LinkedIn at www.linkedin.com/company/bd1/ and Twitter @BDandCo.
Contacts:
|
Media: Mela Sera, APR BD IDS Global Communications 443.824.8012 |
Investors: Francesca DeMartino SVP, Head of Investor Relations |
i American Cancer Society. Cancer Facts & Figures 2022. Atlanta, Ga: American Cancer Society; 2022.
ii American College of Obstetricians and Gynecologists. Updated guidelines for management of cervical cancer screening abnormalities. Practice Advisory. Washington, DC: American College of Obstetricians and Gynecologists; 2020. Available at: https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/10/updated-guidelines-for-management-of-cervical-cancer-screening-abnormalities. Retrieved April 12, 2021
iii Perkins RB, Guido RS, Castle PE, Chelmow D, Einstein MH, Garcia F, et al. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. 2019 ASCCP Risk-Based Management Consensus Guidelines Committee [published erratum appears in J Low Genit Tract Dis 2020;24:427]. J Low Genit Tract Dis 2020;24:102–31. Available at: https://journals.lww.com/jlgtd/Fulltext/2020/04000/2019_ASCCP_Risk_Based_Management_Consensus.3.aspx. Retrieved April 12, 2021
iv National Cancer Institute (NCI). Cancer Stat Facts: cervical cancer. NCI website. https://seer.cancer.gov/statfacts/html/cervix.html
v American Cancer Society. Cancer Facts & Figures 2019. Atlanta, Ga: American Cancer Society; 2019.
SOURCE BD (Becton, Dickinson and Company)